22+ nernst equation calculator
Easy is good so we basically want to force the quadratic equation into the form xa²x²2axa². The following is the procedure how to use the Nernst equation calculator Step 1.
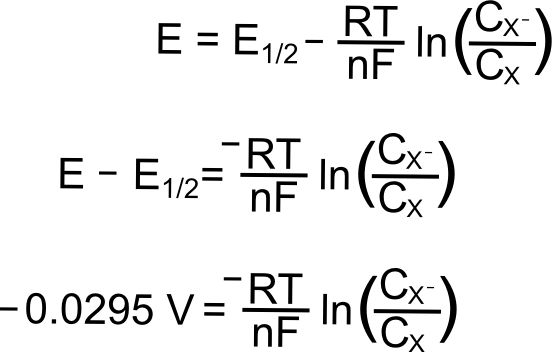
The Nernst Equation
The Nernst equation can be used to calculate.

. The Nernst equation for 298 Kelvin can be represented as follows. The Nernst equation calculates the equilibrium potential also referred to as the Nernst potential for an ion based on the charge on the ion ie its valence and its. The Nernst equation describes the relationship between electrode potential and solution concentration.
The emf and the standard emf of a cell in the following reaction is 5V and 506V at room temperature Ni s. However in non-standard conditions the Nernst equation is used to. Standard cell potentials are calculated in standard conditions of temperature and pressure.
This voltage is often. The equation may be re-arranged to allow calculation of the emf from a known reference concentration and a measured oxygen concentration. In the input field enter the standard half-cell reduction potential chemical activity for the reductant and oxidant.
Nernst equation calculator for converting between oxygen and voltage at a set temperature. In the equation below Q is the reaction quotient and is. K c 322 10 30.
These solutions are compliant with the latest edition books CBSE syllabus and NCERT guidelines. E -00496 T log 10 pO. In electrochemistry the Nernst equation is a chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction half-cell or full cell reaction from the.
Nernst Equation Calculator solved by our expert teachers for academic year 2021-22. The following example shows how the Nernst equation may be used to calculate the potential of an electrochemical cell at non-standard conditions. The Nernst equation is an equation that relates the reduction potential of.
All it takes is making sure that the coefficient of the highest power x² is one. Tutor Pace offer students help with Nernst Equation Calculator for any grade in any subject including math algebra trigonometry and geometry. The critical distance of a FRET process is the distance at which the energy transfer has 50 efficiency half of the energy of the donors relaxation is used by the acceptor.
The Nernst equation can be used to calculate the equilibrium potential for an ion based on its charge valence and its concentration on each side of a membrane. E E0 00592n log_10 Q Hence as per the Nernst equation the potential of the electrochemical cell depends on the.
The Nernst Equation

Statistics In Analytical Chemistry Excel
Nernst Equation Calculator Calculator Academy
How To Calculate And Solve For Nernst Equation Corrosion Nickzom Blog

10 Best Free Online Nernst Equation Calculator Websites

Nernst Equation Concepts Derivation Conditions Example And Videos

Calculating Cell Potentials In Nonstandard Conditions Chemistry Study Com
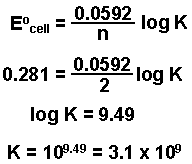
Equilibrium Constant Free Energy

Neet Chemistry Notes Electrochemistry Nernst Equation Cbse Tuts
Write Nernst Equation And Calculate The Emf Of The Following Cell At 298 K Sarthaks Econnect Largest Online Education Community
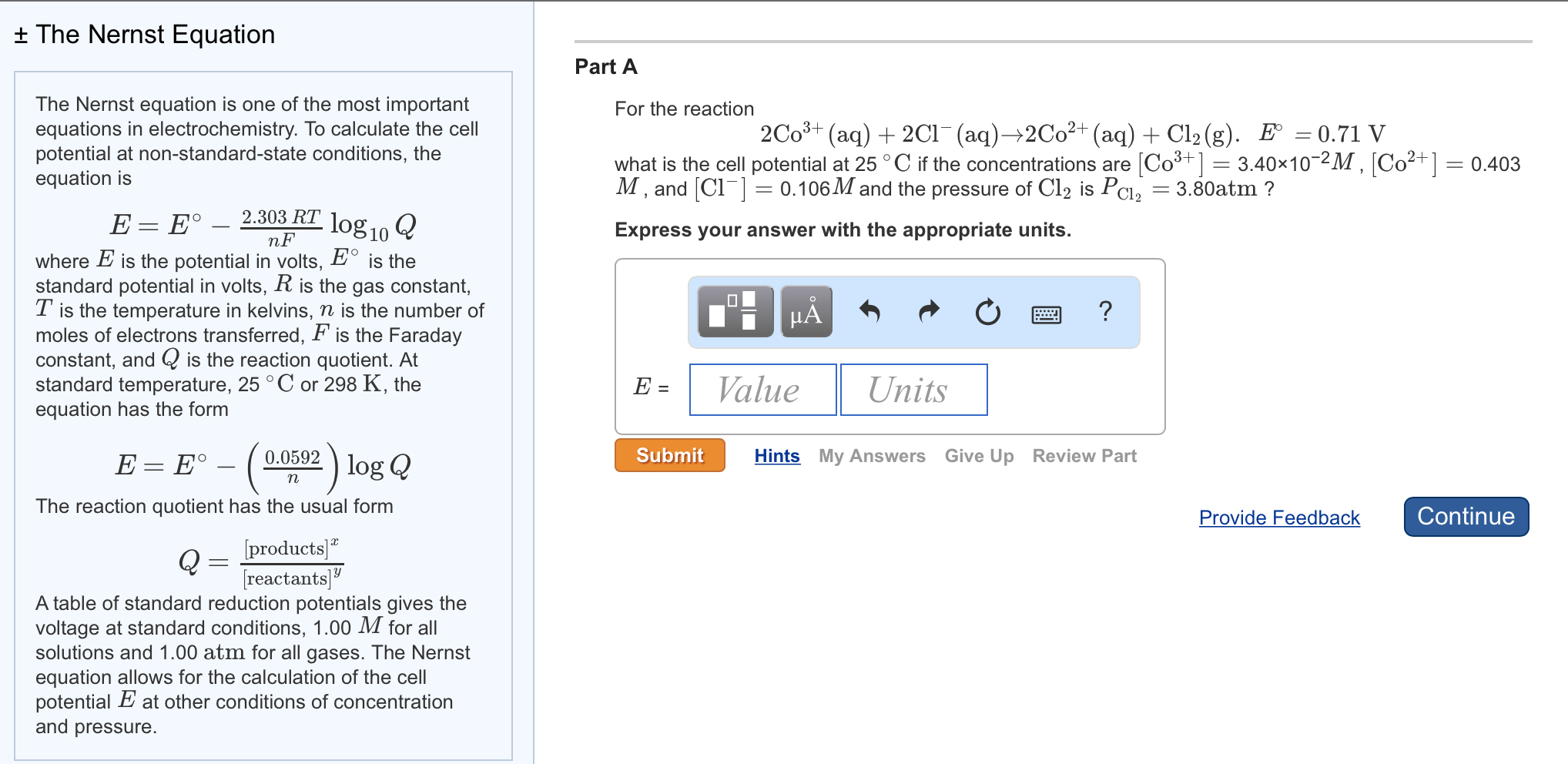
Solved T The Nernst Equation The Nernst Equation Is One Of Chegg Com

Give Nernst Equation Calculate The Electrode Potential Of The Following Single Electrode Cu Aq C 0 01m Cu E 0 337 V

20 6 The Nernst Equation Chemistry Libretexts
What Is The Nernst Equation And Its Application Quora

Nernst Equation Questions Practice Questions Of Nernst Equation With Answer Explanations

Neet Chemistry Notes Electrochemistry Nernst Equation Cbse Tuts
What Is The Nernst Equation Quora